Current Research
Histone Tail Proteolysis
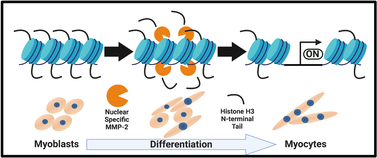
Proteolysis of the histone H3 N-terminal tail (H3NT) is an evolutionarily conserved event in eukaryotic developmental programs, including myogenesis, that produces a clipped histone H3 (H3cl) within chromatin that is retained in ~5-10% of the genome. Despite the discovery of H3NT proteolysis nearly 60 years ago, the biological significance of this epigenetic modification remains largely unknown. It is frequently an epigenetic feature in development, having been observed in the differentiation of embryonic stem cells and multipotent progenitor cells in mouse, humans, and yeast. The Rice labs' current aim is to study this irreversible epigenetic modification further, using the C2C12 myoblast differentiation model. In the Rice labs' recent publication in Epigenetics and Chromatin (See here), we demonstrated that H3NT proteolysis occurs in multiple models of skeletal myogenesis, and that there is a progressive accumulation of a single H3cl product bound within the chromatin. We identified that canonical extracellular matrix protease, matrix metalloproteinase 2 (MMP-2), is the principal h3NT protease of myoblast differentiation and cleaves H3 between K18/Q19. RNAi-mediated depletion of MMP-2 revealed an impairment of H3NT proteolysis and defective myogenic gene activation and differentiation. The Rice lab is continuing to explore the role and mechanism of MMP-2 mediated H3NT proteolysis in myogenesis, and working to identify and study H3NT proteolysis in other cellular systems.
Check out our latest publication: "MMP-2 is a novel histone H3 N-terminal protease necessary for myogenic gene activation"
https://epigeneticsandchromatin.biomedcentral.com/articles/10.1186/s13072-021-00398-4
Proteolysis of the histone H3 N-terminal tail (H3NT) is an evolutionarily conserved event in eukaryotic developmental programs, including myogenesis, that produces a clipped histone H3 (H3cl) within chromatin that is retained in ~5-10% of the genome. Despite the discovery of H3NT proteolysis nearly 60 years ago, the biological significance of this epigenetic modification remains largely unknown. It is frequently an epigenetic feature in development, having been observed in the differentiation of embryonic stem cells and multipotent progenitor cells in mouse, humans, and yeast. The Rice labs' current aim is to study this irreversible epigenetic modification further, using the C2C12 myoblast differentiation model. In the Rice labs' recent publication in Epigenetics and Chromatin (See here), we demonstrated that H3NT proteolysis occurs in multiple models of skeletal myogenesis, and that there is a progressive accumulation of a single H3cl product bound within the chromatin. We identified that canonical extracellular matrix protease, matrix metalloproteinase 2 (MMP-2), is the principal h3NT protease of myoblast differentiation and cleaves H3 between K18/Q19. RNAi-mediated depletion of MMP-2 revealed an impairment of H3NT proteolysis and defective myogenic gene activation and differentiation. The Rice lab is continuing to explore the role and mechanism of MMP-2 mediated H3NT proteolysis in myogenesis, and working to identify and study H3NT proteolysis in other cellular systems.
Check out our latest publication: "MMP-2 is a novel histone H3 N-terminal protease necessary for myogenic gene activation"
https://epigeneticsandchromatin.biomedcentral.com/articles/10.1186/s13072-021-00398-4